28 years old male presented with history of headache and gradual loss of vision in both eyes for 15 days. It was associated with complaints of nausea and vomiting. His headache was intense on awakening and bending down position. There was no history of unconsciousness, seizures, or change in behavior. He was smoker; he was non vegetarian by diet. There was no significant history of similar illness in his family. There was no significant social and environmental history. On examination he was healthy and well oriented to time, place and person. His visual acuity was 6/12 in both eyes. Pupillary reaction was normal in both eyes. Intraocular pressure was 16 mmHg in both eyes. Slit lamp examination showed normal anterior segment. Extraocular movements were normal and colour vision was normal in both eyes. A dilated fundus examination revealed blurred, elevated disc margin, obliterated cup, dilated tortous vessels and peripapillary hemorrages. There was obscuration of all vessels on the disc. Findings were consistent with papilledema (stage v frisen scale classification) Figure 1. Visual field analysis showed an enlarged blind spot in both eyes. His vital signs and systemic examination were normal.
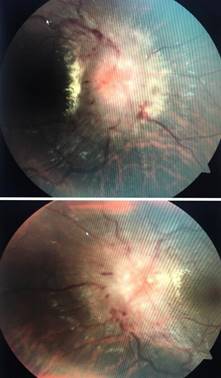
Figure 1: Fundus photograph of right and left eye of patient of neurocysticercosis showing stage v papilledema
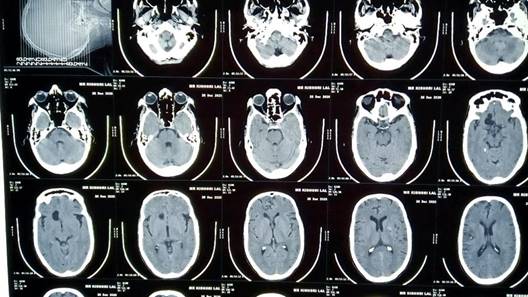
Figure 2: CT scan showing calcified lesion in frontal lobe of brain
Blood reports were hemoglobin 13 gm%, Neutrophils (N) 57%, Lymphocytes (L) 20%, Eosinophils (E) 14%, Monocytes (M) 1%, Erythrocyte Sedimentation Rate (ESR) 30 mm/first hour, Total Leukocyte Count (TLC) 7600 cells/mm3 and Random Blood Sugar (RBS) 110 mg%. His serology was negative. His urine routine microscopy was normal. A CT scan showed calcified lesion in frontal lobe (Figure 2).
A physician consultation was done and he was treated with tablet albendazole 400 bd for 1 month and tablet valproic acid was prescribed 300 mg bd for 1 month. He was kept on follow up.



